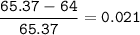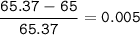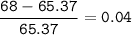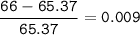Zinc has an average atomic mass of 65.37 amu. Jack is trying to figure out what the most abundant isotope of zinc is, but he doesn't have access to the internet, so the average atomic mass is the only information he has. Jack decides to made an educated guess as to the most abundant isotope of zinc by assuming there are only two isotopes of zinc. If Jack assumes there are only two isotopes of Zn, what he be most likely to decide is zinc's most abundant isotope?
Question 5 options:
Zinc-64
Zinc-65
Zinc-68
Zinc-66

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 23.06.2019 00:00
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3

Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 1
You know the right answer?
Zinc has an average atomic mass of 65.37 amu. Jack is trying to figure out what the most abundant is...
Questions















Advanced Placement (AP), 30.12.2019 22:31

English, 30.12.2019 22:31

Mathematics, 30.12.2019 22:31


English, 30.12.2019 22:31