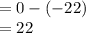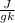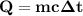At 1 atm, how much energy is required to heat 59.0 g H2O(s) at −22.0 ∘C to H2O(g) at 141.0 ∘C? STRATEGY: Calculate the energy needed for each temperature change or phase change individually. a. The energy needed to heat 59.0 g H2O(s) from −22.0 ∘C to its melting point. b. The energy needed to melt 59.0 g H2O(s) at its melting point. c. The energy needed to heat 59.0 g H2O(l) from the melting point to the boiling point. d. The energy needed to boil 59.0 g H2O(l) at its boiling point. e. The energy needed to heat 59.0 g H2O(g) from the boiling point to 141.0 ∘C. Sum the energies from each step and convert to kilojoules. Step 1. a. Going from −22.0 ∘C to the melting point of H2O (0 ∘C) is a temperature change of 22.0 ∘C. How much energy is needed to heat 59.0 g H2O(s) by 22.0 ∘C if its specific heat is 2.087 J/(g× ∘C)?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:30
The crust of earth may a- continets and ocean floors. b-continents only. c-layers of sedimentary rocks and continents. d-all of the above
Answers: 2

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 05:30
A3.37-mg sample of protein was chemically digested to convert its nitrogen into ammonia and then diluted to 100.0 ml. then 10.0 ml of this solution was placed in a 50-ml volumetric flask and treated with 5 ml of phenol solution plus 2 ml of sodium hypochlorite solution. the sample was diluted to 50.0 ml, and the absorbance at 625 nm was measured in a 1.00-cm cuvette and found to be 0.486. for reference, a standard solution was prepared from 10.0 mg of nh4cl (molar mass = 53.49 grams/mole) dissolved in 1.00 l of water. then 10.0 ml of this standard was placed in a 50-ml volumetric flask, treated in the same manner as the unknown, and the absorbance found to be 0.323. finally, a reagent blank was prepared using distilled water in place of unknown, it was treated in the same manner as the unknown, and the absorbance found to be 0.076. calculate the weight percent of nitrogen in the protein.
Answers: 1

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3
You know the right answer?
At 1 atm, how much energy is required to heat 59.0 g H2O(s) at −22.0 ∘C to H2O(g) at 141.0 ∘C? STRAT...
Questions


History, 15.07.2019 12:20



Chemistry, 15.07.2019 12:20


Physics, 15.07.2019 12:20

Social Studies, 15.07.2019 12:20






Chemistry, 15.07.2019 12:20


Mathematics, 15.07.2019 12:20

Mathematics, 15.07.2019 12:20



Social Studies, 15.07.2019 12:20