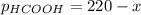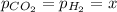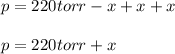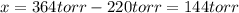Chemistry, 13.10.2020 03:01 91miketaylor
When formic acid is heated, it decomposes to hydrogen and carbon dioxide in a first-order decay: HCOOH(g) →CO2(g) + H2 (g) The rate of reaction is monitored by measuring the total pressure in the reaction container. Time (s) . . . P (torr) 0 . . . . . . . . . 220 50 . . . . . . . . 324 100 . . . . . . . 379 150 . . . . . . . 408 200 . . . . . . . 423 250 . . . . . . . 431 300 . . . . . . . 435 At the start of the reaction (time = 0), only formic acid is present. What is the formic acid pressure (in torr) when the total pressure is 364? Hint: use Dalton's law of partial pressure and the reaction stoichiometry.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 23.06.2019 01:00
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1

Chemistry, 23.06.2019 01:30
If a particle has z = 25 and 23 electrons, what is its charge?
Answers: 2
You know the right answer?
When formic acid is heated, it decomposes to hydrogen and carbon dioxide in a first-order decay: HCO...
Questions



Geography, 11.04.2020 00:53





Mathematics, 11.04.2020 00:53


Arts, 11.04.2020 00:53

Mathematics, 11.04.2020 00:53





Mathematics, 11.04.2020 00:53


History, 11.04.2020 00:53


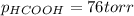
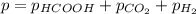
 as the time goes by:
as the time goes by: