
Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change. when the temperature in a room increases from 25°c to 33°c, changes from a solid to a liquid. in a lab, methane and nitrogen are cooled from -170°c to -200°c. the methane freezes and the nitrogen . when gold is heated to 2,856°c it changes from a liquid to a .
Answers: 2

Chemistry, 21.06.2019 21:30
How air particles exert a pressure on the inside of the balloon
Answers: 1

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2
You know the right answer?
Determine the number of atoms of O in 10.0 g of CO2....
Questions

Mathematics, 05.05.2020 10:21




Social Studies, 05.05.2020 10:21

Mathematics, 05.05.2020 10:21


Mathematics, 05.05.2020 10:21




Biology, 05.05.2020 10:21


Mathematics, 05.05.2020 10:21



History, 05.05.2020 10:21

Mathematics, 05.05.2020 10:21

Mathematics, 05.05.2020 10:21

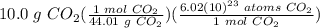 = 1.36787 × 10²³ atoms CO₂
= 1.36787 × 10²³ atoms CO₂

