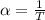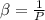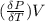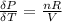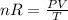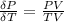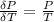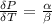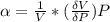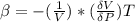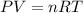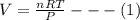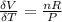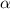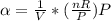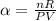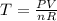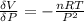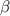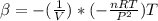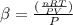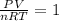The coefficient of thermal expansion α = (1/V)(∂V/∂T)p. Using the equation of state, compute the value of α for an ideal gas. The coefficient of compressibility β is define by β = -(1/V)(∂V/∂p)T. Compute the value of β for an ideal gas. For an ideal gas, express the derivative (∂p/∂T)v in terms of α and β. Do the same derivative for van der Waals gas.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3


You know the right answer?
The coefficient of thermal expansion α = (1/V)(∂V/∂T)p. Using the equation of state, compute the val...
Questions


Mathematics, 07.04.2020 22:39

History, 07.04.2020 22:39

Mathematics, 07.04.2020 22:39

Mathematics, 07.04.2020 22:39

Mathematics, 07.04.2020 22:39



Chemistry, 07.04.2020 22:39








Mathematics, 07.04.2020 22:39


Mathematics, 07.04.2020 22:39