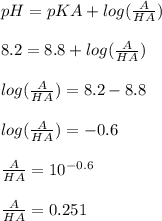Chemistry, 24.09.2020 14:01 because061907
A compound is known to have a free amino group with a pKa of 8.8, and one other ionizable group with a pKa between 5 and 7. To 100 mL of a 0.2 M solution of this compound at pH 8.2 was added 40 mL of a solution of 0.2 M hydrochloric acid. The pH changed to 6.2. The pKa of the second ionizable group is: A) The pH cannot be determined from this information. B) 5.4. C) 5.6. D) 6.0. E) 6.2.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3
You know the right answer?
A compound is known to have a free amino group with a pKa of 8.8, and one other ionizable group with...
Questions



Chemistry, 25.05.2021 17:10


Mathematics, 25.05.2021 17:10


Social Studies, 25.05.2021 17:10


History, 25.05.2021 17:10

Mathematics, 25.05.2021 17:10

English, 25.05.2021 17:10








Physics, 25.05.2021 17:10