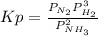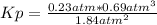Chemistry, 24.09.2020 07:01 20jessicacabriales
Ammonia will decompose into nitrogen and hydrogen at high temperature. An industrial chemist studying this reaction fills a flask with of ammonia gas, and when the mixture has come to equilibrium measures the partial pressure of hydrogen gas to be . Calculate the pressure equilibrium constant for the decomposition of ammonia at the final temperature of the mixture. Round your answer to significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:50
Astudent made a graph plotting the progress of a reaction over time. the student forgot to label the y-axis of the graph. a graph is shown with two graph lines. one graph line starts at a higher position on the y axis and slopes downwards towards the right. the other graph line starts at a lower position on the y axis and slopes upwards towards the right. the two graph lines stop short of intersecting each other and continue as separate lines which gradually become straight and parallel to the x axis. a vertical line is shown at a point where the two graph lines finally became parallel to the x axis. this vertical line is labeled equilibrium. the title on the x axis is time and an arrow pointing towards the right is shown above time. the title on the y axis is left blank. what best explains the label that the student should use on the y-axis? amount, because as the amount of product decreases, the amount of reactant increases over time. reaction rate, because forward and backward reaction become equal at equilibrium. amount, because the amounts of reactants and products become constant after equilibrium is reached. reaction rate, as the rate of forward reaction increases and rate of backward reaction decreases over time.
Answers: 3

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2


Chemistry, 23.06.2019 00:00
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3
You know the right answer?
Ammonia will decompose into nitrogen and hydrogen at high temperature. An industrial chemist studyin...
Questions

Mathematics, 03.11.2020 01:30

Advanced Placement (AP), 03.11.2020 01:30

English, 03.11.2020 01:30


English, 03.11.2020 01:30

Mathematics, 03.11.2020 01:30


Chemistry, 03.11.2020 01:30


Arts, 03.11.2020 01:30


Physics, 03.11.2020 01:30

Mathematics, 03.11.2020 01:30

History, 03.11.2020 01:30

Arts, 03.11.2020 01:30



History, 03.11.2020 01:30

Biology, 03.11.2020 01:30

History, 03.11.2020 01:30