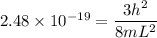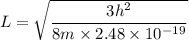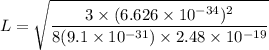When metallic sodium is dissolved in liquid sodium chloride, electrons are released into the liquid. These dissolved electrons absorb light with a wavelength near 800. nm. Suppose we treat the positive ions surrounding an electron crudely as defining a three-dimensional cubic box of edge , and we assume that the absorbed light excites the electron from its ground state to the first excited state. Calculate the edge length in this simple model.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2

Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2
You know the right answer?
When metallic sodium is dissolved in liquid sodium chloride, electrons are released into the liquid....
Questions




Mathematics, 12.07.2019 20:40








Social Studies, 12.07.2019 20:40

English, 12.07.2019 20:40







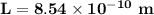
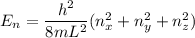
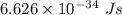
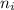 = the quantum no in a specified direction
= the quantum no in a specified direction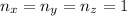
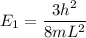
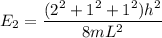
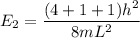
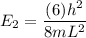
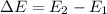
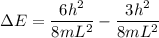
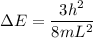 ----- (1)
----- (1)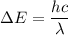
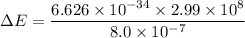
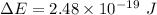
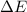 in (1); then:
in (1); then: