
Chemistry, 22.09.2020 20:01 leannamat2106
A pair of 100mL samples of water are taken from a well bored into a large underground salt NaCl deposit. Sample #1 is from the top of the well, and is initially at 32°C. Sample #2 is from a depth of 50.m, and is initially at 42°C. Both samples are allowed to come to room temperature (20.°C) and 1atm pressure. An NaCl precipitate is seen to form in Sample #1.
a. A bigger mass of NaCl precipitate will form in Sample
b. A smaller mass of NaCl precipitate will form in Sample
c. The same mass of NaCl precipitate will form in Sample #2.
d. No, precipitate will form in Sample #2.
e. I need more information to predict whether and how much precipitate will form in Sample #2.

Answers: 2
Another question on Chemistry




Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1
You know the right answer?
A pair of 100mL samples of water are taken from a well bored into a large underground salt NaCl depo...
Questions


Mathematics, 11.10.2020 14:01

Mathematics, 11.10.2020 14:01


Mathematics, 11.10.2020 14:01




Mathematics, 11.10.2020 14:01

English, 11.10.2020 14:01

Mathematics, 11.10.2020 14:01



Mathematics, 11.10.2020 14:01



Mathematics, 11.10.2020 14:01



Mathematics, 11.10.2020 14:01

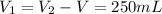
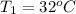
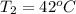
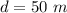
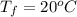
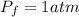
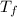 there will be a greater decrease in temperature in sample 2 than in sample 1 so more precipitate of NaCl will be formed in sample 2 than in sample one
there will be a greater decrease in temperature in sample 2 than in sample 1 so more precipitate of NaCl will be formed in sample 2 than in sample one 

