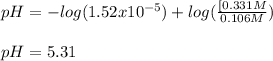A 1.41 L buffer solution consists of 0.149 M butanoic acid and 0.288 M sodium butanoate. Calculate the pH of the solution following the addition of 0.061 moles of NaOH . Assume that any contribution of the NaOH to the volume of the solution is negligible. The Ka of butanoic acid is 1.52×10−5 .

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1


Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2
You know the right answer?
A 1.41 L buffer solution consists of 0.149 M butanoic acid and 0.288 M sodium butanoate. Calculate t...
Questions


Mathematics, 14.12.2020 23:10


Mathematics, 14.12.2020 23:10




History, 14.12.2020 23:10






Physics, 14.12.2020 23:10


Mathematics, 14.12.2020 23:10




Mathematics, 14.12.2020 23:10

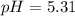
![pH=pKa+log(\frac{[base]}{[acid]} )](/tpl/images/0771/3232/33848.png)
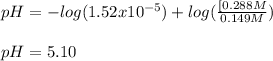
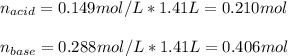
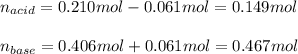
![[acid]=\frac{0.149mol}{1.41L} =0.106M](/tpl/images/0771/3232/f69f4.png)
![[base]=\frac{0.467mol}{1.41L} =0.331M](/tpl/images/0771/3232/b5538.png)