

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:20
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2(g)→2alcl3(s) what is the maximum mass of aluminum chloride that can be formed when reacting 32.0 g of aluminum with 37.0 g of chlorine? express your answer to three significant figures and include the appropriate units.
Answers: 2

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3
You know the right answer?
Nitrosyl bromide decomposes according to the chemical equation below. 2NOBr(g) 2NO(g) + Br2(g) 1.00...
Questions




















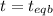 1( 1-2x) 2x x
1( 1-2x) 2x x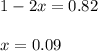
![K_{eq}=\dfrac{[NO]^2[br_2]}{[NOBr]^2}\\\\K_{eq}=\dfrac{0.18^2\times 0.9}{0.82^2}\\\\K_{eq}=0.043\ atm](/tpl/images/0772/5104/fe2ee.png)



