

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1
You know the right answer?
For the reaction 3C2H2(g)---> C6H6(l) at 25 C the standard enthalpy change is -631 kj and the sta...
Questions


Mathematics, 04.08.2019 02:30

Social Studies, 04.08.2019 02:30

English, 04.08.2019 02:30


Computers and Technology, 04.08.2019 02:30

History, 04.08.2019 02:30


History, 04.08.2019 02:30

Biology, 04.08.2019 02:30

Biology, 04.08.2019 02:30



History, 04.08.2019 02:30

Biology, 04.08.2019 02:30

Biology, 04.08.2019 02:30


Business, 04.08.2019 02:30

History, 04.08.2019 02:30

History, 04.08.2019 02:30

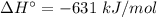 .
.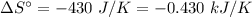 .
.![\Delta G^o=\Delta H^o-T\Delta S^o\\\\\Delta G^o=-631-[298\times (-0.430)]\ kJ\\ \\\Delta G^o=-631-(-128.14)\ kJ\\\\\Delta G^o=-502.86\ kJ](/tpl/images/0770/6490/cfc98.png)


