
Chemistry, 10.09.2020 03:01 CoreyHammond1517
When 229.0 J of energy is supplied as heat to 3.00 mol of Ar(g) at constant pressure the temperature of the sample increases by 2.55 K. Assuming that in the experiment the gas behaves as an ideal gas, calculate the molar heat capacities at constant volume and at constant pressure of Ar(g).

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1

Chemistry, 22.06.2019 23:00
If two identical atoms are bonded,what kind of molecule is formed
Answers: 1

Chemistry, 22.06.2019 23:30
To find the work done, the force exerted and distance moved are multiplied. a couch is moved twice before you are happy with its placement. the same force was used to move the couch both times. if more work is done the first time it is moved, what do you know about the distance it was moved? a) when more work was done, the couch was moved the same distance. b) when more work was done, the couch was moved less. c) when more work was done, the couch was moved further. d) when more work was done, the couch wasn't moved at all.
Answers: 1

You know the right answer?
When 229.0 J of energy is supplied as heat to 3.00 mol of Ar(g) at constant pressure the temperature...
Questions


Biology, 09.09.2019 01:20

English, 09.09.2019 01:20


Biology, 09.09.2019 01:20

English, 09.09.2019 01:20

Mathematics, 09.09.2019 01:20

History, 09.09.2019 01:20




Computers and Technology, 09.09.2019 01:20

History, 09.09.2019 01:20





Biology, 09.09.2019 01:20

Mathematics, 09.09.2019 01:20

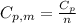
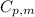 is the molar heat capacity at constant pressure
is the molar heat capacity at constant pressure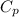 is the heat capacity at constant pressure
is the heat capacity at constant pressure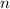 is the number of moles
is the number of moles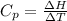
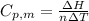
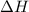 = 229.0 J
= 229.0 J = 2.55 K
= 2.55 K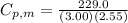
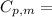 29.93 JK⁻¹mol⁻¹
29.93 JK⁻¹mol⁻¹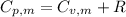
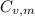 is the molar heat capacity at constant volume
is the molar heat capacity at constant volume 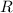 is the gas constant (
is the gas constant (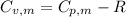
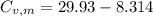
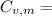 21.62 JK⁻¹mol⁻¹
21.62 JK⁻¹mol⁻¹

