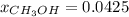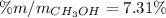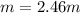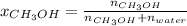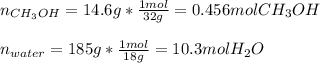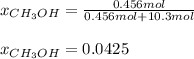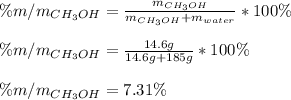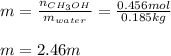Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:20
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2
You know the right answer?
A solution is made containing 14.6g of CH3OH in 185g H2O.1. Calculate the mole fraction of CH3OH.2....
Questions

Mathematics, 14.07.2020 16:01

Advanced Placement (AP), 14.07.2020 16:01

Biology, 14.07.2020 17:01

Mathematics, 14.07.2020 17:01



Mathematics, 14.07.2020 17:01

English, 14.07.2020 17:01

Biology, 14.07.2020 17:01

Mathematics, 14.07.2020 17:01

Mathematics, 14.07.2020 17:01


Mathematics, 14.07.2020 17:01

Mathematics, 14.07.2020 17:01


Mathematics, 14.07.2020 17:01

Mathematics, 14.07.2020 17:01



Computers and Technology, 14.07.2020 17:01