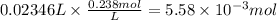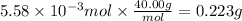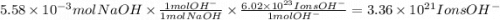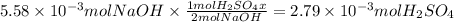Chemistry, 08.09.2020 14:01 amandaestevez030
Consider a 0.238 M aqueous solution of sodium hydroxide, NaOH.
a. How many grams of NaOH are dissolved in 23.46 mL?
b. How many individual hydroxide ions (OH) are found in 23.46 mL?
c. How many moles of sulfuric acid, H2SO4, are neutralized by 23.46 mL of 0.238 M NaOH(aq)?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1


Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1
You know the right answer?
Consider a 0.238 M aqueous solution of sodium hydroxide, NaOH.
a. How many grams of NaOH are dissol...
Questions

Medicine, 30.11.2020 02:20



Social Studies, 30.11.2020 02:30


Mathematics, 30.11.2020 02:30

Mathematics, 30.11.2020 02:30

Mathematics, 30.11.2020 02:30

Mathematics, 30.11.2020 02:30

Mathematics, 30.11.2020 02:30




History, 30.11.2020 02:30

Mathematics, 30.11.2020 02:30

Computers and Technology, 30.11.2020 02:30


Arts, 30.11.2020 02:30


History, 30.11.2020 02:30