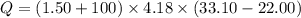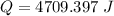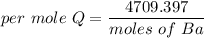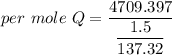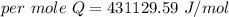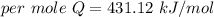Chemistry, 07.09.2020 18:01 breanna7667
When 1.50 g of Ba(s) is added to 100.00 g of water in a container open to the atmosphere, the reaction shown below occurs and the temperature of the resulting solution rises from 22.00°C to 33.10°C. If the specific heat of the solution is 4.18 J/(g ∙ °C), calculate for the reaction, as written. Ba(s) + 2 H2O(l) → Ba(OH)2(aq) + H2(g)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:30
Covalent network solids typically have melting points and boiling points. the chemical formula of a network solid indicates in the molecule.
Answers: 3

Chemistry, 21.06.2019 20:50
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1

Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1
You know the right answer?
When 1.50 g of Ba(s) is added to 100.00 g of water in a container open to the atmosphere, the reacti...
Questions


Spanish, 12.12.2020 16:50


Mathematics, 12.12.2020 16:50


Biology, 12.12.2020 16:50



English, 12.12.2020 16:50

Mathematics, 12.12.2020 16:50




Mathematics, 12.12.2020 16:50


Mathematics, 12.12.2020 16:50

Mathematics, 12.12.2020 16:50

Mathematics, 12.12.2020 16:50

Spanish, 12.12.2020 16:50

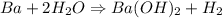
 temperature
temperature