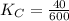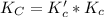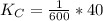Chemistry, 06.09.2020 02:01 Deavionaaaaa
Given the equilibrium constants for the following two reactions at a 298K:NiO(s) + H2(g) ⇌ Ni(s) + H2O(g) Kc=40NiO(s) +CO(g) ⇌ Ni(s) +CO2(g) Kc=600Calculate the value for the equilibrium constant, Kc, for the reaction:CO2(g) + H2(g) ⇌ CO(g) + H2O(g)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2

Chemistry, 23.06.2019 01:10
Volume is a measurement of how fast particles of a substance are moving
Answers: 3

Chemistry, 23.06.2019 02:50
Select the correct location on the image identify the element that humans need to breathe. 2015 er r ights reserved
Answers: 3
You know the right answer?
Given the equilibrium constants for the following two reactions at a 298K:NiO(s) + H2(g) ⇌ Ni(s) + H...
Questions

Mathematics, 12.09.2019 06:20

Biology, 12.09.2019 06:20


Mathematics, 12.09.2019 06:20




Engineering, 12.09.2019 06:20



Mathematics, 12.09.2019 06:20

Chemistry, 12.09.2019 06:20


History, 12.09.2019 06:20



Mathematics, 12.09.2019 06:20

Law, 12.09.2019 06:20

English, 12.09.2019 06:20

Spanish, 12.09.2019 06:20