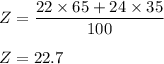Chemistry, 05.09.2020 23:01 jaimevalenzuela60
Consider an element Z that has two naturally occuring isotopes with the following percent abundances: the isotope with a mass number 22.0 is 65.0% abundant; the isotope with a mass number 24.0 is 35.0% abundant. What is the average atomic mass for element Z? Round your answer to the hundredth.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2


Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3
You know the right answer?
Consider an element Z that has two naturally occuring isotopes with the following percent abundances...
Questions

Social Studies, 30.01.2020 16:02



Chemistry, 30.01.2020 16:02

Mathematics, 30.01.2020 16:02

Mathematics, 30.01.2020 16:02

Health, 30.01.2020 16:02



Mathematics, 30.01.2020 16:02



History, 30.01.2020 16:02


Mathematics, 30.01.2020 16:02



History, 30.01.2020 16:02

Mathematics, 30.01.2020 16:02