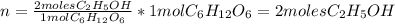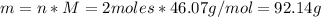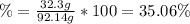Chemistry, 02.09.2020 04:01 BabyRage4900
A 1.00-mole sample of C6H12O6 was placed in a vat with 100 g of yeast. If 32.3 grams of C2H5OH was obtained, what was the percent yield of C2H5OH?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1

Chemistry, 22.06.2019 19:20
For a research project, a student decided to test the effect of the lead(ii) ion (pb2+) on the ability of salmon eggs to hatch. this ion was obtainable from the water‐soluble salt, lead(ii) nitrate, which the student decided to make by the following reaction. pbo(s) + 2 hno3(aq) → pb(no3)2(aq) + h2o losses of product for various reasons were expected, and a yield of 86.0% was expected. in order to have 5.00 g of product at this yield, how many grams of pbo should be reacted? (assume that sufficient nitric acid, hno3, would be used.)
Answers: 1

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2
You know the right answer?
A 1.00-mole sample of C6H12O6 was placed in a vat with 100 g of yeast. If 32.3 grams of C2H5OH was o...
Questions



History, 01.08.2019 07:30


Social Studies, 01.08.2019 07:30



Biology, 01.08.2019 07:30





History, 01.08.2019 07:30



Mathematics, 01.08.2019 07:30



Mathematics, 01.08.2019 07:30