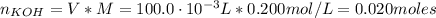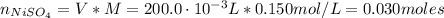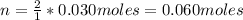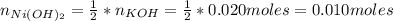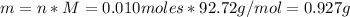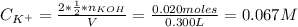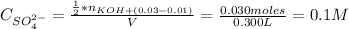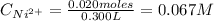A solution of 100.0 mL of 0.200 M KOH is mixed with a solution of 200.0 mL of 0.150 M NiSO4. (a) Write the balanced chemical equation for the reaction that occurs. (b) What precipitate forms? (c) What is the limiting reactant? (d) How many grams of this precipitate form? (e) What is the concentration of each ion that remains in solution?

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 15:30
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1
You know the right answer?
A solution of 100.0 mL of 0.200 M KOH is mixed with a solution of 200.0 mL of 0.150 M NiSO4. (a) Wri...
Questions

English, 19.03.2021 08:10

Mathematics, 19.03.2021 08:10

Mathematics, 19.03.2021 08:10



English, 19.03.2021 08:10

Social Studies, 19.03.2021 08:10


Chemistry, 19.03.2021 08:10

History, 19.03.2021 08:10


Arts, 19.03.2021 08:10



History, 19.03.2021 08:10




Mathematics, 19.03.2021 08:10