
Chemistry, 28.08.2020 20:01 eshaesmot12345
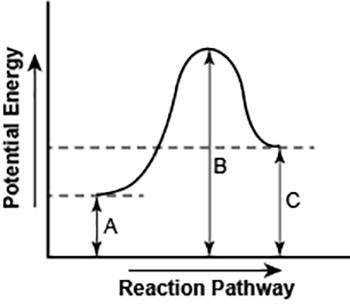
Part 1: Describe how you can determine the total change in enthalpy and activation energy from the diagram, and if each is positive or negative. Part 2: Describe how the curve will look if the reaction was exothermic. Be sure to mention changes in the potential energies of the reactants and products and the sign changes of the enthalpy.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2
You know the right answer?
Part 1: Describe how you can determine the total change in enthalpy and activation energy from the d...
Questions


Mathematics, 11.11.2020 06:10

Mathematics, 11.11.2020 06:10

History, 11.11.2020 06:10

Mathematics, 11.11.2020 06:10




Biology, 11.11.2020 06:10

Biology, 11.11.2020 06:10

Advanced Placement (AP), 11.11.2020 06:10


Advanced Placement (AP), 11.11.2020 06:10







Mathematics, 11.11.2020 06:10



