
Chemistry, 27.08.2020 21:01 oksanabkrot
A reaction between 7.0 g of copper(II) oxide and 50 mL of 0.20 M nitric acid produces
copper(II) nitrate, Cu(NO3)2 and water.
(c) Determine the limiting reactant.
(d) Calculate the mass of excess reactant after the reaction.
(ANS: 6.6068g)
(e) Determine the percentage yield if the actual mass of copper (II) nitrate obtained from
the reaction is 0.85 g.
(ANS: 90.64%)
How to get the mass of HNO3 from here? I only managed to get mass of NO3 based on the molarity formula. thanks!

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:00
What is the molar mass of babr2? a. 217.2 g/mol b. 297.1 g/mol c. 354.5 g/mol d. 434.4 g/mol
Answers: 1

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2

Chemistry, 22.06.2019 23:00
Which organism develops breathing organism develops breathing organs from pharyngeal arches? shark, spider, sea star, sea horse
Answers: 2
You know the right answer?
A reaction between 7.0 g of copper(II) oxide and 50 mL of 0.20 M nitric acid produces
copper(II) ni...
Questions


Computers and Technology, 17.07.2021 14:00


Mathematics, 17.07.2021 14:00

English, 17.07.2021 14:00



Health, 17.07.2021 14:00

Health, 17.07.2021 14:00

Computers and Technology, 17.07.2021 14:00

Chemistry, 17.07.2021 14:00

Social Studies, 17.07.2021 14:00

English, 17.07.2021 14:00

Physics, 17.07.2021 14:00

Physics, 17.07.2021 14:00

English, 17.07.2021 14:00

Mathematics, 17.07.2021 14:00

English, 17.07.2021 14:00

Mathematics, 17.07.2021 14:00

English, 17.07.2021 14:00

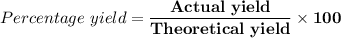
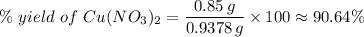
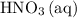 is the limiting reactant.
is the limiting reactant.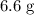 of
of 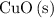 will be in excess.
will be in excess.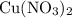 is approximately
is approximately 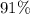 . (Rounded to two significant figures, as in other quantities in the question.)
. (Rounded to two significant figures, as in other quantities in the question.)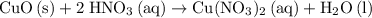 .
.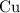 :
: 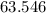 .
. :
: 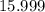 .
.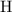 :
: 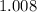 .
.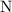 :
: 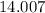 .
.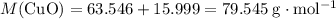 .
.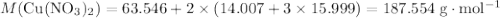 .Limiting Reactant
.Limiting Reactant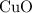 and
and 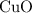 is the limiting one. In other words, assume that all the
is the limiting one. In other words, assume that all the 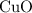 is consumed before
is consumed before 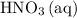 was.
was. 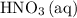 would be required to convert all that
would be required to convert all that 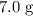 of
of 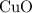 to
to 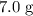 of
of 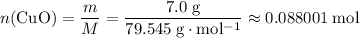 .
.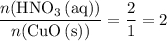 .
.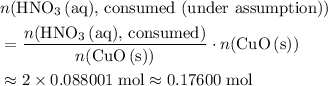 .
. 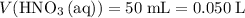 .
.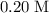 solution:
solution: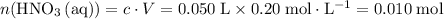 .
.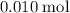 of
of 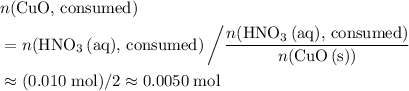 .
.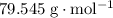 . Therefore, the mass of that
. Therefore, the mass of that 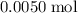 formula units of
formula units of 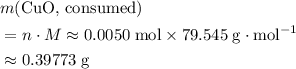 .
.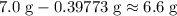 of
of 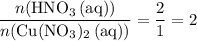 .
.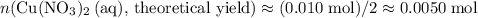 .
.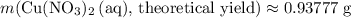 .
.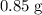 :
: .
.

