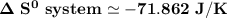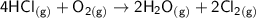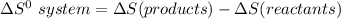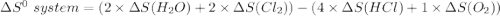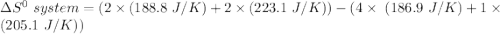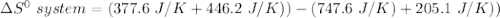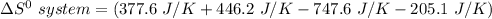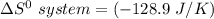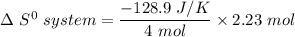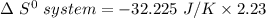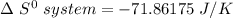Chemistry, 26.08.2020 15:01 rayvingrant16
Consider the reaction: 4HCl(g) + O2(g)2H2O(g) + 2Cl2(g) Using standard absolute entropies at 298K, calculate the entropy change for the system when 2.23 moles of HCl(g) react at standard conditions. S°system = J/K

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 05:30
Idont understand it 1.what is the boiling point of a solution of 675 grams of ethylene glycol (c2h6o2) in 2.50 liters of water?
Answers: 2

Chemistry, 23.06.2019 08:00
What is the temperature in kelvin of a gas if it is allowed to expand from 1.50 l to 4.50 l? the initial temperature is 10.0°c and pressure is constant throughout the change. which equation should you use? t2= v2/v1 t1 what is the final temperature? ⇒ 849 k these are the answers.
Answers: 1

Chemistry, 23.06.2019 11:00
The image below shows a weather service map.. i’m not sure if is correct
Answers: 2

Chemistry, 23.06.2019 14:00
During an acid-base titration, when do the contents of the beaker consist of only water, a salt, and a trace of indicator?
Answers: 2
You know the right answer?
Consider the reaction: 4HCl(g) + O2(g)2H2O(g) + 2Cl2(g) Using standard absolute entropies at 298K, c...
Questions

History, 05.11.2020 21:10





Chemistry, 05.11.2020 21:10





History, 05.11.2020 21:10


Mathematics, 05.11.2020 21:10





Mathematics, 05.11.2020 21:10

Chemistry, 05.11.2020 21:10