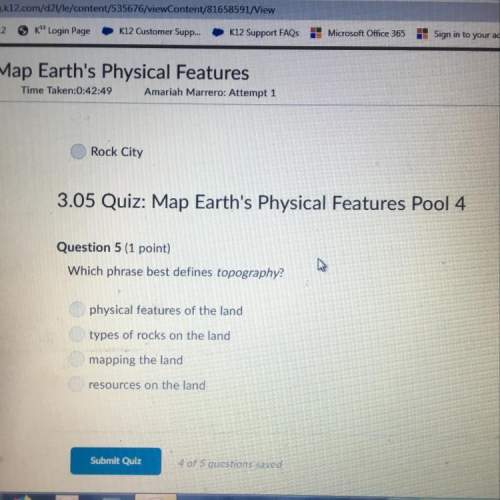
Chemistry, 20.08.2020 16:01 freedygotmoney
Nvm solved
1. A solution contains 0.0450 M Ca2+ and 0.0930 M Ag+. If solid Na3PO4 is added to this mixture, which of the phosphate species would precipitate out of solution first? It is Ca3(PO4)2 2.
2. When the second cation just starts to precipitate, what percentage of the first cation remains in solution? percentage = ? %

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 22.06.2019 22:30
Which of the following is true about the speed of light? it depends on the wavelength.
Answers: 3

Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3

Chemistry, 23.06.2019 02:40
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 latex: \longrightarrow ⟶ co2(g) latex: \delta δ h° = –393.5 kj/mol h2(g) + o2 latex: \longrightarrow ⟶ h2o(l) latex: \delta δ h° = –285.8 kj/mol ch3oh(l) + o2(g) latex: \longrightarrow ⟶ co2(g) + 2h2o(l) latex: \delta δ h° = –726.4 kj/mol
Answers: 3
You know the right answer?
Nvm solved
1. A solution contains 0.0450 M Ca2+ and 0.0930 M Ag+. If solid Na3PO4 is added to this...
Questions


Health, 19.09.2019 02:40

Biology, 19.09.2019 02:40

Chemistry, 19.09.2019 02:40

Advanced Placement (AP), 19.09.2019 02:40



Mathematics, 19.09.2019 02:40




Social Studies, 19.09.2019 02:40

Mathematics, 19.09.2019 02:40


Mathematics, 19.09.2019 02:40




Mathematics, 19.09.2019 02:40