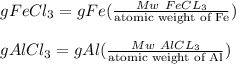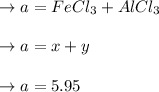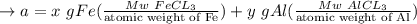Chemistry, 15.08.2020 23:01 dameiranderson
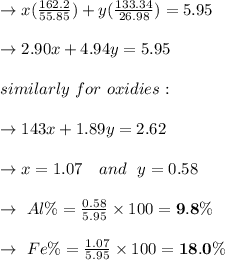
A mixture containing only FeCl3 and AlClz weighs 5.95 g . The chlorides are converted to the hydrous oxides and ignited to Fe2O3 and Al2O3 . The oxide mixture weighs 2.62 g . Calculate the percent Fe and Al in the original mixture .

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 00:00
How many peaks will be present in a mass spectrum for brcl?
Answers: 1

Chemistry, 23.06.2019 03:10
Which of the following compounds would be expected to have the strongest ionic bonds? a)the compound that has b)the largest ions with the greatest charge c)the compound that has d)the largest ions with the least charge the compound that has the smallest ions with the greatest charge the compound that has the smallest ions with the least charge
Answers: 2

Chemistry, 23.06.2019 03:30
Which of the following describes the entropy change as a solution is made from a liquid and solid
Answers: 1

Chemistry, 23.06.2019 06:30
Aplanet similar to earth has four moons roughly the same distance away. the moon that will most affect tides on the planet is the one that has the greatest a) mass. b) volume. c) density. d) amount of water.
Answers: 1
You know the right answer?
A mixture containing only FeCl3 and AlClz weighs 5.95 g . The chlorides are converted to the hydrous...
Questions

History, 16.11.2019 14:31


Mathematics, 16.11.2019 14:31

Mathematics, 16.11.2019 14:31



Physics, 16.11.2019 14:31







Mathematics, 16.11.2019 14:31



Biology, 16.11.2019 14:31


Mathematics, 16.11.2019 14:31

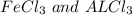 = 5.95g
= 5.95g