
Chemistry, 15.08.2020 08:01 kingofmortals2119
Why is calcium chloride (rock salt, CaCl2) more effective than sodium chloride (table salt, NaCl) at de-icing roads in the winter? A. Calcium chloride only breaks into 2 ions, while sodium chloride does not break up and remains 1 ion. B. Calcium is a larger molecule than sodium, so it has a greater effect. C. The additional chlorine atom forms a stronger bond between the atoms in calcium chloride than in sodium chloride, so calcium chloride remains one molecule while sodium chloride breaks apart. D. Calcium chloride breaks into 3 ions, while sodium chloride only breaks into 2 ions.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Complete this brønsted-lowry reaction placing each product by its appropriate label. hso4- + hcn
Answers: 1

Chemistry, 21.06.2019 19:00
State the formula for density in words and mathematical symbols
Answers: 2

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1
You know the right answer?
Why is calcium chloride (rock salt, CaCl2) more effective than sodium chloride (table salt, NaCl) at...
Questions



Mathematics, 11.11.2019 18:31


History, 11.11.2019 18:31





History, 11.11.2019 18:31

Physics, 11.11.2019 18:31


Mathematics, 11.11.2019 18:31

History, 11.11.2019 18:31


English, 11.11.2019 18:31


Social Studies, 11.11.2019 18:31

Chemistry, 11.11.2019 18:31

Spanish, 11.11.2019 18:31

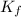 × m × i
× m × i

