
Chemistry, 13.08.2020 18:01 sampurple123
Dinitrogen tetroxide and hydrazine (N2H4) undergo a redox reaction in which nitrogen and water are formed as products. What mass of nitrogen could be produced when 50.0 g of dinitrogen tetroxide and 45.0 g of hydrazine are combined?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2
You know the right answer?
Dinitrogen tetroxide and hydrazine (N2H4) undergo a redox reaction in which nitrogen and water are f...
Questions

Mathematics, 29.09.2020 01:01


Mathematics, 29.09.2020 01:01




Business, 29.09.2020 01:01

Mathematics, 29.09.2020 01:01



Mathematics, 29.09.2020 01:01


English, 29.09.2020 01:01

Advanced Placement (AP), 29.09.2020 01:01


Spanish, 29.09.2020 01:01



English, 29.09.2020 01:01

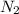 = 45.65 g
= 45.65 g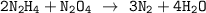
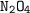 :
: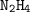 :
:

