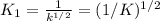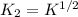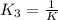Chemistry, 14.08.2020 05:01 ramireznaidelyn
Methanol liquid burns readily in air. One way to represent this equilibrium is: 2 CO2(g) + 4 H2O(g)2 CH3OH(l) + 3 O2(g) We could also write this reaction three other ways, listed below. The equilibrium constants for all of the reactions are related. Write the equilibrium constant for each new reaction in terms of K, the equilibrium constant for the reaction above. 1) CH3OH(l) + 3/2 O2(g) CO2(g) + 2 H2O(g) K1 = 2) CO2(g) + 2 H2O(g) CH3OH(l) + 3/2 O2(g) K2 = 3) 2 CH3OH(l) + 3 O2(g) 2 CO2(g) + 4 H2O(g)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2

Chemistry, 23.06.2019 01:00
Chromium(iii) sulfate is a transition metal compound containing the metal chromium and the polyatomic ion sulfate. the oxidation state of chromium in this compound is , and the chemical formula of the compound is ( ) . reset next
Answers: 3

Chemistry, 23.06.2019 01:30
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
Methanol liquid burns readily in air. One way to represent this equilibrium is: 2 CO2(g) + 4 H2O(g)2...
Questions



Chemistry, 25.01.2020 00:31




Law, 25.01.2020 00:31


Geography, 25.01.2020 00:31

Mathematics, 25.01.2020 00:31



Social Studies, 25.01.2020 00:31





Mathematics, 25.01.2020 00:31

Physics, 25.01.2020 00:31

Mathematics, 25.01.2020 00:31