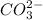Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 23.06.2019 02:00
The point along a planet's orbit where it is closest to the sun is called the
Answers: 1

Chemistry, 23.06.2019 07:00
Ajar contains a certain substance. which observation would show that the substance must be either a solid or a liquid?
Answers: 1

Chemistry, 23.06.2019 10:40
Aliquid solution can be made select all that apply. dissolving solids into liquids, mixing liquids, dissolving gas solutes into liquids , mixing gases, mixing solids
Answers: 3
You know the right answer?
g Which ONE of the following molecules and ions has trigonal planar molecular geometry? (NOTE: You m...
Questions



English, 27.01.2020 18:31

Computers and Technology, 27.01.2020 18:31




Mathematics, 27.01.2020 18:31

Chemistry, 27.01.2020 18:31



Biology, 27.01.2020 18:31

Mathematics, 27.01.2020 18:31

Mathematics, 27.01.2020 18:31

Biology, 27.01.2020 18:31