Chemistry, 13.08.2020 04:01 ziyonsolange
A sample of radioactive silver contains two isotopes, 108Ag (denoted A) and 110Ag (denoted B). The second of these (B) has a half life of 24 seconds, whereas the first (A) has a half life of 2.3 minutes. If a sample contains equal numbers of each of these isotopes at the beginning of an experiment that runs for an hour, which of the following statements is correct?
A. At the end of the hour, isotope B has a greater decay constant λ than isotope A
B. At the end of the hour, isotope A has the same decay constant λ as isotope B
C. At the end of the hour, isotope A has a greater decay constant λ than isotope B

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 20:00
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3

Chemistry, 23.06.2019 00:00
How many peaks will be present in a mass spectrum for brcl?
Answers: 1

Chemistry, 23.06.2019 02:00
What can be done to make a solid solute dissolve faster in a liquid solvent?
Answers: 1

Chemistry, 23.06.2019 03:10
Which is true according to the law of conservation of energy
Answers: 1
You know the right answer?
A sample of radioactive silver contains two isotopes, 108Ag (denoted A) and 110Ag (denoted B). The s...
Questions


Mathematics, 25.05.2021 02:00

Mathematics, 25.05.2021 02:00



History, 25.05.2021 02:00

Health, 25.05.2021 02:00

Mathematics, 25.05.2021 02:00



Business, 25.05.2021 02:00

Mathematics, 25.05.2021 02:00





Mathematics, 25.05.2021 02:00

Chemistry, 25.05.2021 02:00


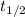 = 0.693/λ
= 0.693/λ