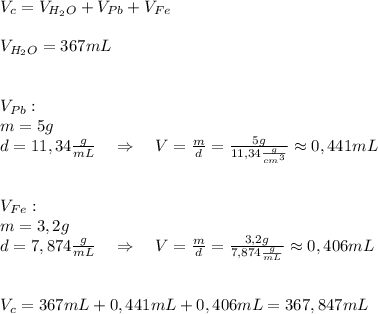A5.0 gram sample of lead and a 3.2 g sample of iron are placed into 367 ml of water. what will be the new volume level of water in units of ml? the density of lead is 11.34 g/cc and the density of iron is 7.874 g/ml. round your answer to three significant figures

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 23.06.2019 01:40
Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide as shown below. caco3(s) cao(s) + co2(g) the kp for this reaction is 1.16 at 800°c. a 5.00 l vessel containing 10.0 g of caco3(s) was evacuated to remove the air, sealed, and then heated to 800°c. ignoring the volume occupied by the solid, what will be the mass of the solid in the vessel once equilibrium is reached?
Answers: 1
You know the right answer?
A5.0 gram sample of lead and a 3.2 g sample of iron are placed into 367 ml of water. what will be th...
Questions

Mathematics, 19.10.2019 18:30

Social Studies, 19.10.2019 18:30

Mathematics, 19.10.2019 18:30



Biology, 19.10.2019 18:30

Geography, 19.10.2019 18:30




Biology, 19.10.2019 18:30


History, 19.10.2019 18:30


Mathematics, 19.10.2019 18:30

Mathematics, 19.10.2019 18:30


Mathematics, 19.10.2019 18:30


Mathematics, 19.10.2019 18:30