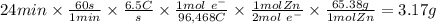Chemistry, 12.08.2020 06:01 sparkybig12
Zinc is used as a coating for steel to protect the steel from environmental corrosion. If a piece of steel is submerged in an electrolysis bath for 24 minutes with a current of 6.5 Amps, how many grams of zinc will be plated out? The molecular weight of Zn is 65.38, and Zn+2 + 2e– → Zn. Question 7 options: A) 3.17 g of Zn B) 1.09 g of Zn C) 6.34 g of Zn D) 12.68 g of Zn

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which feature do highland climates have that lower elevation areas do not?
Answers: 1

Chemistry, 22.06.2019 02:00
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

You know the right answer?
Zinc is used as a coating for steel to protect the steel from environmental corrosion. If a piece of...
Questions




Mathematics, 18.02.2020 19:46


World Languages, 18.02.2020 19:46




Mathematics, 18.02.2020 19:46





Business, 18.02.2020 19:47

World Languages, 18.02.2020 19:47


Mathematics, 18.02.2020 19:47