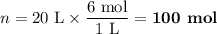I need some help with a long chemistry problem. Anything is appreciated!
You arrive at a crime scene and are told the body of the victim is in the 10 m deep pool. You walk over to what you are told is a 12 m in diameter, cylindrical pool. From the outside of the pool, you can see that the body looks badly burnt. Your partner says, “It looks like our victim had been burned alive and tried to put out the fire by jumping in the pool. The victim likely drowned to death.”
Something does not sit right with you though. If there was a fire, where did it start? There are no signs of combustion anywhere. You aren’t so sure and ask the crime scene investigator to run a sample of the pool water before letting anybody try to pull the body out.
The CSI comes back to you and tells you that normal pool water pH is roughly around 7.2, but the pool pH is actually highly basic at a level of 13 with a concentration of hydroxide ions at 1.0 x 10-1 mol/L. It becomes obvious to you that the body wasn’t burned before going in to the pool , but AFTER and there was no fire needed!
You order the body to be removed from the pool, but the CSI interjects, “It would be too dangerous with a pH that high. I suggest you get some vinegar from the store and pour it in to the pool before hand to drop the pH to 7. Draining the pool would take far too long and we need to examine the body as soon as possible.” She asks one of your constables to go to the store to purchase 5 – 4L jugs of vinegar (pH = 2) to pour in the pool, as she states it is enough to bring the pH to a safe level of 7.
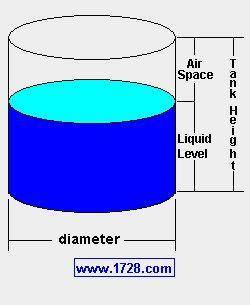
It sounds like it could be enough vinegar based on your knowledge of acids and bases, but you want to double-check her estimate before sending your constable to the store. Verify whether or not she is correct using calculations. A diagram may help you with your calculations

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2

Chemistry, 22.06.2019 21:30
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3

Chemistry, 23.06.2019 02:00
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1
You know the right answer?
I need some help with a long chemistry problem. Anything is appreciated!
You arrive at a crime scen...
Questions

Physics, 16.09.2019 12:50

English, 16.09.2019 12:50

History, 16.09.2019 12:50



Health, 16.09.2019 12:50

Mathematics, 16.09.2019 12:50



Biology, 16.09.2019 12:50

Mathematics, 16.09.2019 12:50







Social Studies, 16.09.2019 12:50

Computers and Technology, 16.09.2019 12:50

![K_{\text{a}} = \dfrac{\text{[A]}^{-}\text{[H$_{3}$O$^{+}$]}}{\text{[HA]}} = 1.76 \times 10^{-5}](/tpl/images/0715/4396/ee3a6.png)
![\begin{array}{rcl}\dfrac{\text{[A]}^{-}\text{[H$_{3}$O$^{+}$]}}{\text{[HA]}}& = & 1.76 \times 10^{-5}\\\\\dfrac{0.01\times 0.01}{c}& = & 1.76 \times 10^{-5}\\\\1 \times 10^{-4} & = & 1.76 \times 10^{-5}c\\c & = & \dfrac{1 \times 10^{-4}}{1.76 \times 10^{-5}}\\\\ & = & \text{6 mol/L}\\\end{array}](/tpl/images/0715/4396/b069d.png)