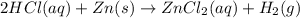Chemistry, 24.07.2020 09:01 aprilpendergrass
9. In a certain chemical reaction, 2 hydrogen chloride molecules in aqueous solution react with solid zinc. The reaction produces zinc
chloride in aqueous solution and hydrogen gas. Which of the following reaction equations correctly describes this reaction?
A. 2HCI (g) + 2Zn (s) - 2ZnCl2 (s) + H2 (9)
B. HCI (aq) + 2Zn (s) - 2ZnCl (aq) + H2 (9)
C. 2HCl (aq) + Zn (s) → ZnCl2 (aq) + H2 (g)
D. HCI (aq) + Zn (s) Zn, Cl (aq) + 2H2 (1)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 18:00
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2
You know the right answer?
9. In a certain chemical reaction, 2 hydrogen chloride molecules in aqueous solution react with soli...
Questions



Computers and Technology, 01.07.2021 20:00






Business, 01.07.2021 20:00








Mathematics, 01.07.2021 20:00