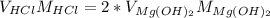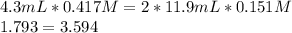Chemistry, 18.07.2020 08:01 juanitarodrigue
It was calculated that 4.3mL of 0.417 M HCl is required to titrate 11.9 mL of 0.151 M Mg(OH)2. Show evidence 2 HCl(aq) + Mg(OH)2(aq) → + MgCl2(aq) + 2 H2O(l)

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3


Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1
You know the right answer?
It was calculated that 4.3mL of 0.417 M HCl is required to titrate 11.9 mL of 0.151 M Mg(OH)2. Show...
Questions

Business, 30.01.2020 12:00

Mathematics, 30.01.2020 12:00


Arts, 30.01.2020 12:00


Mathematics, 30.01.2020 12:00


Mathematics, 30.01.2020 12:00





English, 30.01.2020 12:00



Spanish, 30.01.2020 12:01


Mathematics, 30.01.2020 12:01