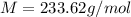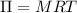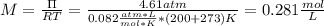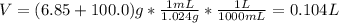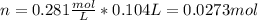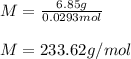Chemistry, 17.07.2020 22:01 SoccerdudeDylan
A solution of 6.85g of a carbohydrate in 100.0g of water has a density of 1.024 g/mL and an osmotic pressure of 4.61 atm at 200C. Calculate molar mass of the carbohydrate

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:20
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2(g)→2alcl3(s) what is the maximum mass of aluminum chloride that can be formed when reacting 32.0 g of aluminum with 37.0 g of chlorine? express your answer to three significant figures and include the appropriate units.
Answers: 2

Chemistry, 21.06.2019 20:00
Smog is the term used to describe the combination of fog and smoke
Answers: 1

Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1
You know the right answer?
A solution of 6.85g of a carbohydrate in 100.0g of water has a density of 1.024 g/mL and an osmotic...
Questions







Mathematics, 03.08.2020 14:01


Mathematics, 03.08.2020 14:01








Mathematics, 03.08.2020 14:01