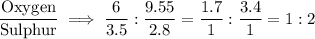Chemistry, 17.07.2020 21:01 maybrieldridge12
Two oxide of sulphur, A and B were analyzed and the results obtained showed that in oxide A,3.50g of sulphur combined with 6.00g of oxygen and in oxide B,2.80g of sulphur combined with 9.55g. Show that this result illustrate the law of multiple proportion

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 22.06.2019 12:00
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3
You know the right answer?
Two oxide of sulphur, A and B were analyzed and the results obtained showed that in oxide A,3.50g of...
Questions


Mathematics, 26.10.2020 16:00

Physics, 26.10.2020 16:00


Mathematics, 26.10.2020 16:00

Mathematics, 26.10.2020 16:00

Advanced Placement (AP), 26.10.2020 16:00

Mathematics, 26.10.2020 16:00

Mathematics, 26.10.2020 16:00


Advanced Placement (AP), 26.10.2020 16:00

Social Studies, 26.10.2020 16:00


Social Studies, 26.10.2020 16:00





Mathematics, 26.10.2020 16:00

English, 26.10.2020 16:00