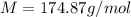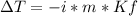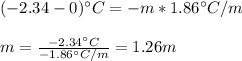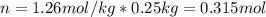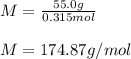Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2
You know the right answer?
A solution that contains 55.0 g of ascorbic acid (Vitamin C) in 250. g of water freezes at –2.34°C....
Questions

Mathematics, 30.07.2019 18:00

English, 30.07.2019 18:00



History, 30.07.2019 18:00




Geography, 30.07.2019 18:00

Mathematics, 30.07.2019 18:00


Biology, 30.07.2019 18:00

History, 30.07.2019 18:00



Mathematics, 30.07.2019 18:00


Spanish, 30.07.2019 18:00