
Chemistry, 16.07.2020 17:01 payshencec21
The equilibrium constant, Kc, for the following reaction is 55.6 at 698 K:
H2(g) + I2(g) 2HI(g)
Calculate the equilibrium concentrations of reactants and product when 0.234 moles of H2 and 0.234 moles of I2 are introduced into a 1.00 L vessel at 698 K.
[H2] = M
[I2] = M
[HI] = M

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 13:30
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1
You know the right answer?
The equilibrium constant, Kc, for the following reaction is 55.6 at 698 K:
H2(g) + I2(g) 2HI(g)
Questions

Social Studies, 19.12.2019 03:31





Mathematics, 19.12.2019 03:31

Spanish, 19.12.2019 03:31

Mathematics, 19.12.2019 03:31


Chemistry, 19.12.2019 03:31

Biology, 19.12.2019 03:31


Mathematics, 19.12.2019 03:31

Mathematics, 19.12.2019 03:31


English, 19.12.2019 03:31


Mathematics, 19.12.2019 03:31


Mathematics, 19.12.2019 03:31

![[I_2]=[H_2]=0.369M](/tpl/images/0707/8736/ed0a2.png)
![[HI]=0.0495M](/tpl/images/0707/8736/4fca5.png)
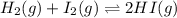
![Kc=\frac{[HI]^2}{[I_2][H_2]}](/tpl/images/0707/8736/bf8a4.png)
 (considering the ICE procedure) is written as:
(considering the ICE procedure) is written as:![55.6=\frac{(2x)^2}{([I_2]_0-x)([H_2]_0-x)}](/tpl/images/0707/8736/e5efd.png)
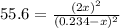
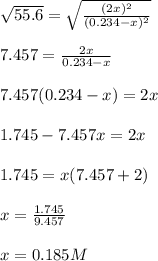
![[I_2]=[H_2]=2*0.185M=0.369M](/tpl/images/0707/8736/ec590.png)
![[HI]=0.234-0.185=0.0495M](/tpl/images/0707/8736/318f7.png)


