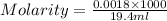Chemistry, 15.07.2020 18:01 giselabarajas24
Sulfamic acid, HSO3NH2 (molar mass = 97.1 g/mol), is a strong monoprotic acid that can be used to standardize a strong base: A 0.179-g sample of HSO3NH2 required 19.4 mL of an aqueous solution of KOH for a complete reaction. What is the molarity of the KOH solution?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:20
What is the strongest intermolecular force between an nacl unit and an h2o molecule together in a solution? covalent bonding dipole-dipole force hydrogen bonding ion-dipole force
Answers: 1

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1


Chemistry, 23.06.2019 08:30
According to the passage, which of these is true about gray water systems? a) gray water systems use plants that require less water. eliminate b) gray water systems require the use of less fossil fuels. c) gray water systems reduce the amount of fresh water used. d) gray water systems reduce the amount water used by shower heads.
Answers: 1
You know the right answer?
Sulfamic acid, HSO3NH2 (molar mass = 97.1 g/mol), is a strong monoprotic acid that can be used to st...
Questions


Mathematics, 15.11.2021 08:20

Computers and Technology, 15.11.2021 08:30

English, 15.11.2021 08:30

Mathematics, 15.11.2021 08:40



Mathematics, 15.11.2021 09:00


Mathematics, 15.11.2021 09:00






Mathematics, 15.11.2021 09:10

Mathematics, 15.11.2021 09:20



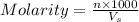
 = volume of solution in ml
= volume of solution in ml =
= 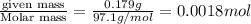
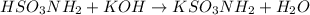
 moles of KOH
moles of KOH