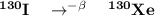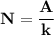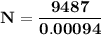Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1



Chemistry, 23.06.2019 08:00
Determine the number of moles of air present in 1.35 l at 750 torr and 17.0°c. which equation should you use? n=pv/rt what is the number of moles present? ⇒ 0.056 mol a sample of n2 gas occupying 800.0 ml at 20.0°c is chilled on ice to 0.00°c. if the pressure also drops from 1.50 atm to 1.20 atm, what is the final volume of the gas? which equation should you use? v2= p1v1t2/p2t1 what is the final volume of the gas? ⇒ 932 ml these are the answers
Answers: 1
You know the right answer?
130I decays by emission of beta particles to form stable 130Xe. A 3.00 g iodine sample containing so...
Questions

English, 14.04.2021 17:20



Mathematics, 14.04.2021 17:20

Health, 14.04.2021 17:20


Mathematics, 14.04.2021 17:20

English, 14.04.2021 17:20

Mathematics, 14.04.2021 17:20

Mathematics, 14.04.2021 17:20


Mathematics, 14.04.2021 17:20


Physics, 14.04.2021 17:20




Mathematics, 14.04.2021 17:20

Mathematics, 14.04.2021 17:20