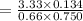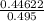When H2O and CO react at 979°C, the products are CO2 and H2. The equilibrium constant (in terms of equilibrium concentrations of reactants and products) for the reaction below is 0.66 at 979°C. If the following concentrations are measured after the reaction reaches equilibrium, what is the concentration of CO(g) in the equilibrated mixture? answer will be in M
Component: Measured Equilibrium Concentration
A. H2 0 (g) 0.750 M
B. CO2 (g) 0.134 M
C. H2 (g) 3.33 M

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1



Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1
You know the right answer?
When H2O and CO react at 979°C, the products are CO2 and H2. The equilibrium constant (in terms of e...
Questions

Arts, 01.03.2021 14:00

History, 01.03.2021 14:00


Mathematics, 01.03.2021 14:00

History, 01.03.2021 14:00


Mathematics, 01.03.2021 14:00

Biology, 01.03.2021 14:00

Chemistry, 01.03.2021 14:00

Mathematics, 01.03.2021 14:00


Chemistry, 01.03.2021 14:00


History, 01.03.2021 14:00


Mathematics, 01.03.2021 14:00




Mathematics, 01.03.2021 14:00

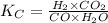
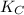 = equilibrium constant
= equilibrium constant