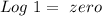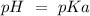Chemistry, 09.07.2020 01:01 jybuccaneers2022
50 mL of 0.1 M acetic acid is mixed with 50 mL of 0.1 M sodium acetate (the conjugate base). The Ka of acetic acid is approximately 1. 74 X 10 -5. What is the pH of the resulting solution?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 23.06.2019 01:00
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2
You know the right answer?
50 mL of 0.1 M acetic acid is mixed with 50 mL of 0.1 M sodium acetate (the conjugate base). The Ka...
Questions









Engineering, 12.08.2020 18:01








Computers and Technology, 12.08.2020 18:01


Biology, 12.08.2020 18:01

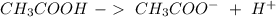
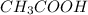 ) and a base (
) and a base (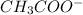 ). Therefore we can write the henderson-hasselbach reaction:
). Therefore we can write the henderson-hasselbach reaction:![pH~=~pKa+Log\frac{[CH_3COO^-]}{[CH_3COOH]}](/tpl/images/0703/3490/99062.png)
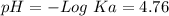
![[CH_3COO^-]=[CH_3COOH]](/tpl/images/0703/3490/ee54c.png)
![\frac{[CH_3COO^-]}{[CH_3COOH]}~=~1](/tpl/images/0703/3490/6e489.png)