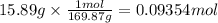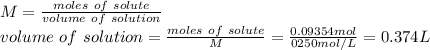A student wants to make a 0.250 M aqueous solution of silver nitrate, AgNO3, and has a bottle containing 15.89 g of silver nitrate. What should be the final volume of the solution? When you give your numerical answer, what is the correct significant figures and how do you know that is the correct amount?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 22.06.2019 10:00
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1


Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3
You know the right answer?
A student wants to make a 0.250 M aqueous solution of silver nitrate, AgNO3, and has a bottle contai...
Questions

Geography, 04.12.2019 02:31

Arts, 04.12.2019 02:31

Mathematics, 04.12.2019 02:31




Social Studies, 04.12.2019 02:31


Mathematics, 04.12.2019 02:31


Biology, 04.12.2019 02:31

Mathematics, 04.12.2019 02:31

Computers and Technology, 04.12.2019 02:31

History, 04.12.2019 02:31


Geography, 04.12.2019 02:31




Social Studies, 04.12.2019 02:31