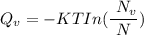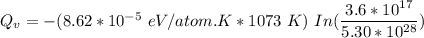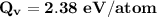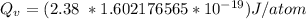Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 23.06.2019 04:20
The lewis diagrams for magnesium and fluorine are shown below. what is the correct chemical formula for magnesium fluoride? a. mgf b. mg2f c. mgf2 d. mg2f3
Answers: 1

Chemistry, 23.06.2019 07:00
How does science use models to gain a better understanding of concepts?
Answers: 1
You know the right answer?
Calculate the energy (in J/atom) for vacancy formation in silver, given that the equilibrium number...
Questions




Mathematics, 24.02.2020 23:46

Mathematics, 24.02.2020 23:46





Physics, 24.02.2020 23:46

Mathematics, 24.02.2020 23:46

Mathematics, 24.02.2020 23:46



Physics, 24.02.2020 23:47

Mathematics, 24.02.2020 23:47

Mathematics, 24.02.2020 23:47



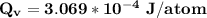
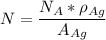
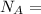 avogadro's number =
avogadro's number = 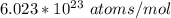
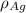 = Density of silver = 9.5 g/cm³
= Density of silver = 9.5 g/cm³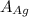 = Atomic weight of sliver = 107.9 g/mol
= Atomic weight of sliver = 107.9 g/mol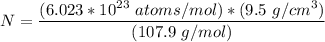
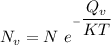
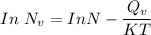
 the subject of the formula; we have:
the subject of the formula; we have: