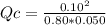The reaction of nitrogen gas and oxygen gas to form nitrogen monoxide gas is shown below. If the measured concentrations of all three chemicals at some point in time are: [N2] = 0.80 M
[O2] = 0.050 M
[NO] = 0.10 M
Which statement is TRUE about the reaction at this point in time? N2(g) + O2(g) ⇄ 2 NO(g) K = 0.10
The reaction is at equilibrium.
The reverse reaction is occurring at a faster rate than the forward reaction.
The forward reaction is occurring at a faster rate than the reverse reaction.
This set of concentration values is impossible because the concentrations of N2 and O2 must be the same.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2


Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1
You know the right answer?
The reaction of nitrogen gas and oxygen gas to form nitrogen monoxide gas is shown below. If the mea...
Questions


Chemistry, 21.04.2021 16:40

Mathematics, 21.04.2021 16:40

Mathematics, 21.04.2021 16:40

English, 21.04.2021 16:40

History, 21.04.2021 16:40

Biology, 21.04.2021 16:40

History, 21.04.2021 16:40

Mathematics, 21.04.2021 16:40

Mathematics, 21.04.2021 16:40

Arts, 21.04.2021 16:40

Physics, 21.04.2021 16:40

Physics, 21.04.2021 16:40

Mathematics, 21.04.2021 16:40


Mathematics, 21.04.2021 16:40


Mathematics, 21.04.2021 16:40

Social Studies, 21.04.2021 16:40

![Qc=\frac{[C]^{c}*[D]^{d} }{[A]^{a}*[B]^{b} }](/tpl/images/0700/8667/038b9.png)
![Qc=\frac{[NO]^{2} }{[N_{2} ]*[O_{2} ] }](/tpl/images/0700/8667/47464.png)