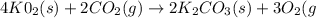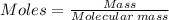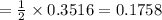Chemistry, 02.07.2020 15:01 MalikaJones
A self-contained breathing apparatus can be a life-saving piece of personal protective equipment (PPE) for first responders. SCBA provide breathing oxygen in a contained system for use in low-oxygen environments or in the presence of toxic fumes. The oxygen is generated through the reaction of potassium superoxide, KO2 and carbon dioxide, forming potassium carbonate and oxygen gas. If the 25.0 g KO2 in the SCBA system was exposed to 45.0 g CO2, what scenario best describes the outcome:

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1
You know the right answer?
A self-contained breathing apparatus can be a life-saving piece of personal protective equipment (PP...
Questions

Chemistry, 15.02.2022 08:40

Mathematics, 15.02.2022 08:40


Computers and Technology, 15.02.2022 08:50







Mathematics, 15.02.2022 08:50


Chemistry, 15.02.2022 08:50




Social Studies, 15.02.2022 08:50


Business, 15.02.2022 08:50