Chemistry, 02.07.2020 23:01 graciewilson
Consider the insoluble compound silver bromide , AgBr . The silver ion also forms a complex with ammonia . Write a balanced net ionic equation to show why the solubility of AgBr (s) increases in the presence of ammonia and calculate the equilibrium constant for this reaction. For Ag(NH3)2+ , Kf = 1.6×107 . Use the pull-down boxes to specify states such as (aq) or (s).

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Balance this equation: n2 + h2 > nh3, write the following molar ratios: n2 / n2 / nh3 h2 /
Answers: 1

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2
You know the right answer?
Consider the insoluble compound silver bromide , AgBr . The silver ion also forms a complex with amm...
Questions

Mathematics, 24.10.2020 18:30


Mathematics, 24.10.2020 18:30

Mathematics, 24.10.2020 18:30

Computers and Technology, 24.10.2020 18:30




Geography, 24.10.2020 18:30



Mathematics, 24.10.2020 18:30

Mathematics, 24.10.2020 18:30


Mathematics, 24.10.2020 18:30

English, 24.10.2020 18:30

History, 24.10.2020 18:30

Arts, 24.10.2020 18:30

Mathematics, 24.10.2020 18:30

Mathematics, 24.10.2020 18:30

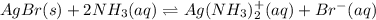
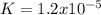
![AgBr(s)\rightleftharpoons Ag^+(aq)+Br^-(aq) \ \ \ Ksp=[Ag^+][Br^-]=7.7x10^{-13}](/tpl/images/0700/1987/811ab.png)
![Ag^+(aq)+2NH_3(aq)\rightleftharpoons Ag(NH_3)_2^+(aq)\ \ \ Kf=\frac{[Ag(NH_3)_2^+]}{[Ag^+][NH_3]^2}=1.6x10^7](/tpl/images/0700/1987/15017.png)
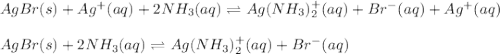
![AgBr(s)+Ag^+(aq)+2NH_3(aq)\rightleftharpoons Ag(NH_3)_2^+(aq)+Br^-+Ag^+\\\\K=[Ag^+][Br^-]*\frac{[Ag(NH_3)_2^+]}{[Ag^+][NH_3]^2}](/tpl/images/0700/1987/20011.png)