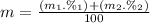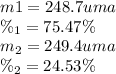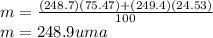Chemistry, 02.10.2019 14:50 Jsquad8879
Calculate the average atomic mas of an unknown element that has two naturally-occurring isotopes.
the first isotope occurs 75.47% of the time and has a mass of 248.7 a. m.u.
the second isotope occurs 24.53% of the time and has a mass of 249.4 a. m.u.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1

Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2

Chemistry, 23.06.2019 01:30
Adirect relationship can be represented by: a curve a pie chart
Answers: 2
You know the right answer?
Calculate the average atomic mas of an unknown element that has two naturally-occurring isotopes.
Questions






Mathematics, 22.10.2019 10:00

English, 22.10.2019 10:00





History, 22.10.2019 10:00



Spanish, 22.10.2019 10:00


Business, 22.10.2019 10:00



History, 22.10.2019 10:00