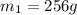Chemistry, 30.06.2020 18:01 6710000831
A silver block, initially at 56.1 ∘C, is submerged into 100.0 g of water at 24.0 ∘C, in an insulated container. The final temperature of the mixture upon reaching thermal equilibrium is 28.0∘C. What is the mass of the silver block?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1
You know the right answer?
A silver block, initially at 56.1 ∘C, is submerged into 100.0 g of water at 24.0 ∘C, in an insulated...
Questions


Mathematics, 24.12.2019 13:31


Mathematics, 24.12.2019 13:31



Geography, 24.12.2019 13:31


Mathematics, 24.12.2019 13:31

Mathematics, 24.12.2019 13:31

English, 24.12.2019 13:31


Chemistry, 24.12.2019 13:31





Mathematics, 24.12.2019 13:31


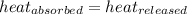
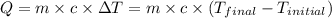
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0697/4196/09236.png) .................(1)
.................(1) = mass of silver = ?
= mass of silver = ? = mass of water = 100.0 g
= mass of water = 100.0 g = final temperature =
= final temperature = 
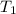 = temperature of silver =
= temperature of silver = 
 = temperature of water =
= temperature of water = 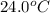
 = specific heat of silver =
= specific heat of silver = 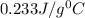
 = specific heat of water=
= specific heat of water= 
![-m_1\times 0.233\times (28.0-56.1)=[100.0\times 4.184\times (28.0-24.0)]](/tpl/images/0697/4196/c0833.png)